The around the world journey of Mutographs samples
Following the Mutographs’ focus on kidney, colorectal and pancreatic cancers, esophageal squamous cell carcinoma and esophageal adenocarcinoma, the co-Principal Investigator at IARC Paul Brennan heads work package 1 to investigate the incidence of these cancers worldwide.
The International Agency for Research on Cancer (IARC), is an intergovernmental institution located in Lyon, France, that forms part of the World Health Organisation. Its role is to coordinate and conduct research into the aetiology of cancer as well as to collect and publish surveillance data on cancer prevalence around the world. For this, it collaborates with research centres, university hospitals or clinical hospitals with associated research units all over the world to collect tumour samples.
Sandra Perdomo, a scientist at IARC, describes the workflow that the samples included in the Mutographs study follow:
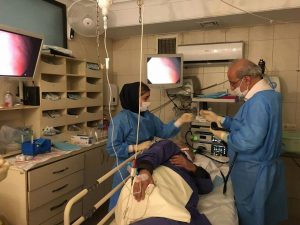
Prof. Malekzadeh with a patient in the Digestive Diseases Research Institute in Tehran, Iran.
In the collaborating centres around the world, cases are identified and a biopsy is taken to confirm diagnosis. The patient is then invited to participate, consent is signed and samples consisting of a blood sample and the remaining tissue of the biopsy are stored. In the cases when surgery is the primary treatment, a piece of the surgical resection is also kept. Information about the patient such as age, gender, habits and risk exposure such as alcohol, tobacco, diet, occupational, etc; as well as clinical data is collected and entered in a centralised online database at IARC. The samples are sent to IARC to be added to its large biobank containing almost 7 million biological samples.

Samples being transported from the Ontario Institute for Cancer Research, Canada
Once at IARC, the samples are inventoried and their corresponding information is curated, verified and stored in the database. The tissue samples and processed and slides are prepared in the histopathology lab. These are reviewed by four pathologists to confirm the tumour type and tissue characteristics. The quality of the tissue is assessed to make sure it contains at least 50% tumour cells and is adequate for sequencing. The suitable tissues are sent to the lab for DNA extraction along with the blood sample from the same patient. The DNA quantity and quality are checked and shipped to the Sanger Institute for sequencing together with core data exclusively concerning the sample, without any specific reference to the patient to preserve privacy.
The Mutographs’ project manager at the Sanger Institute, Laura Humphreys follows on the journey of the samples once they arrive in the UK: At the Sanger Institute the DNA samples undergo further quality control checks and enter the high throughput DNA sequencing pipeline – this requires input from many individuals, teams and also robots and results in high quality sequence data which is then passed on to the Mutographs scientists for detailed analysis. The samples are all given a new unique reference number at Sanger which can be linked to the clinical data, but not to the patient. Ultimately the data from patients’ samples will be presented to the scientific community through publications and conference presentations. We also hope to provide a research update directly to the collaborating medical centres and patients or family members in the final stages of the project.
Over the past months as SARS-Cov2 spread across the world, IARC teams all working remotely, have been following closely the situation in all contributing centres, nearly all of which are health research facilities. In many institutes, much non-essential work was curtailed and patient recruitment slowed down.
The IARC scientist Estelle Chanudet talks about how centres around the world have coped with the local restrictions to continue the work enabling the collection of samples for research:

Restrictions on lab occupancy are imposed in the Ontario Institute for Cancer Research, Canada
At the Ontario Institute for Cancer Research, Canada, Mutographs collaborators began working remotely in mid-March and technical work, such as fine laser cutting of tumour pieces to capture the tumour cells, was on hold. Dr Wilson reported that a small number of lab staff received approval to return to work on June 1st, 2020, with physical distancing measures in place.
South Africa went into lockdown on March 26th, and although this has been eased somewhat since June 1st, Universities are still closed. Dr Parker reported that the University of Cape Town is looking at a phased return of staff and students, weary of the peaking of covid-19 cases. Hospital procedures are limited to only essential surgeries since the Western cape has almost 70% of the Covid-19 incidence and deaths. The Hospital Ethics Committee has ruled that no samples may be collected for research purposes during the lockdown in order to minimise “non-essential” activities and traffic through the hospital.
Across the world, hospitals have been putting in place protocols to ensure patient and health personal safety during recruitment. Collaborating centres in Poland continued recruitment collecting samples whenever possible to avoid close contact of health personnel with patients.

Following new regulations for sample processing at the Hospital Italiano de Buenos Aires, Argentina.
In Argentina, the team led by Dr Vaccaro at the Hospital Italiano de Buenos Aires adapted the Mutographs protocol in order to minimize the risks of contagion while safeguarding the original ethical and legal principles. As such, the enrolment stage of patient identification, initial interview, information about the protocol, signature of informed consent, lifestyle questionnaires and clinical information was conducted exclusively via telephone or video calls and documents were provided electronically. All patients were also tested for Covid-19 before surgery.

Precautions are taken to continue research efforts in the Digestive Diseases Research Institute of Tehran, Iran.
Despite Iran being badly hit by SARS-Cov2, teams in the Digestive Diseases Research Institute in Teheran, Iran, led by Professor Malekzadeh, continued their critical clinical work, also liaising with other Mutographs collaborators across the country.
Similarly, other participating institutions like the National Cancer Institute in Lithuania and Charles University in Prague, Czech Republic, slowly continued recruitment during the epidemic as for many patients critical surgery was necessary and already scheduled.
Other centers like Barretos Cancer hospital and Porto Alegre Hospital in Brazil as well as the International Organization for Cancer Prevention and Research (IOCPR) in Serbia had to stop recruitment for many weeks. Staff were sent back to work remotely and in some collaborators in the project were reassigned to work on Covid-19 specific projects or general clinical assistance.
The situation across all continents continues to be closely monitored thanks to regular feedback from committed collaborators, despite the crisis context. Together with the continuous work completed before the crisis and the re-direction towards data analysis, these efforts continue to drive research forward in hope for a better future for cancer patients and for all of us.